
Harnessing the power of stem cells to repair damaged joints
Every day, the human body is busy sealing cuts, mending micro-damage to our organs, and fighting off infection, keeping our bodies healthy. However, these repair processes can get overwhelmed by injury, inflammation, or illness and can lead to chronic degenerative disease. One example is osteoarthritis, a disease affecting the structures within our joints, particularly the articular cartilage, a smooth, shiny material at the ends of our bones that allows for smooth, frictionless, and pain-free movement. Damage and inflammation can disrupt the smooth cartilage leading to inflammation, pain, stiffness, disability, and overall reduced quality of life. The World Health Organization (WHO) estimates that over 528 million people worldwide are living with osteoarthritis, and, owing to the aging population, as well as the increasing incidence of chronic inflammatory conditions such as obesity, this number is only set to rise in the coming years. Treatment options include pain relief and anti-inflammatory medication but ultimately surgery is required with total joint replacement the only definitive treatment. However, joint replacement surgery is extremely invasive and is not suitable for younger patients as artificial joints have a limited lifespan of 10-20 years and revision surgeries come with additional risks. The restoration of fully functional articular cartilage, with the correct structure and mechanical properties of native tissue has not been achieved to date.
Regenerative medicine aims to harness and stimulate the body’s natural repair response to support healing and encompasses novel treatments such as gene therapy, tissue engineering, and cell therapy. Adult articular cartilage cells, called articular chondrocytes, can be isolated from healthy cartilage and the articular chondrocyte therapy ‘Spherox’ was approved for use in knee cartilage defects in 2017. However, this treatment is limited by the quantity and quality of the patient’s articular chondrocytes, leading to interest in more sustainable sources of cells, namely stem cells. Stem cells have the potential to become many different cell types, including articular chondrocytes. Therefore, theoretically, implanting stem cell-derived chondrocytes could provide an unlimited source of articular cartilage to repair damaged joints.
The source of stem cells is critical to a successful therapy; despite decades of research and 126 ongoing clinical trials, adult mesenchymal stem cells are not ideal as they tend to generate fibrous or bony tissue, rather than smooth articular cartilage. This is because articular cartilage develops before birth and is designed to last for our lifetime. To successfully generate structurally and mechanically accurate articular cartilage, we need to study the stem cells that exist in the very early days of human development: embryonic stem cells (ESCs). ESCs are pluripotent, meaning that they have the potential to become any of the more than 200 different types of cells in the body. However, ethical issues associated with the use of ESCs led scientists to reprogramme adult cells back to an ESC-like pluripotent state, called induced pluripotent stem cells (iPSCs), work that was awarded the Nobel Prize in Physiology or Medicine in 2012. iPSCs represent an unlimited source of cells for regenerative medicine, but, while most scientists agree on the immense regenerative potential of these cells, pluripotency is a double-edged sword; the same ability to turn into hundreds of different cell types, also makes these cells difficult to control.
For pluripotent stem cell therapy to be successful, the cells must first be safely and efficiently directed to become the correct cell type. For cartilage repair, this means they must become stable articular chondrocytes that can regenerate articular cartilage. Our recently published paper in the Journal of Orthopaedic Research tackles the complex path to clinical translation of human pluripotent stem cell (hPSC)-derived articular chondrocytes. We describe a carefully controlled protocol to safely and efficiently turn hPSCs into stable articular chondrocytes. The resulting articular chondrocytes could be expanded several times, each time significantly increasing the quantity of cells, without affecting their ability to produce cartilage tissue. Furthermore, the hPSC-derived articular chondrocytes could consistently regenerate articular cartilage tissue with the correct structure and mechanical properties. A major challenge in the clinical translation of cell therapies is the large‐scale manufacture of cells with the desired properties, therefore, the ability to freeze and store (cryopreserve) these cells has important practical and economic advantages. We report no loss in cell viability or regenerative potential following cryopreservation. These results support the immense potential of hPSC‐derived articular chondrocytes and an important future step for more precise and effective treatments for injured and diseased articular cartilage.
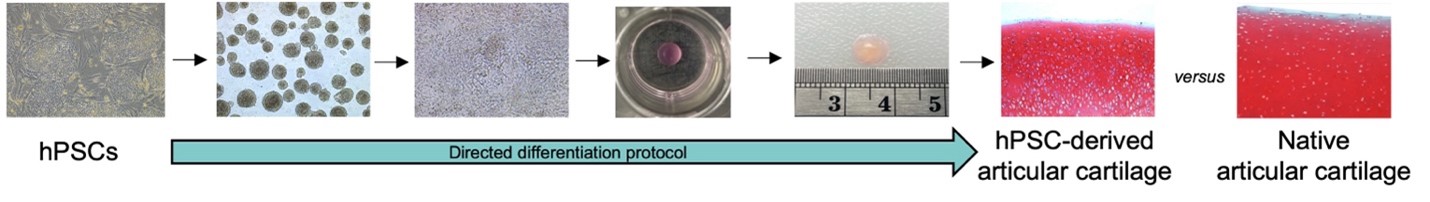
This research was completed while I was working as a research fellow at Harvard Medical School and Boston Children’s Hospital. The work was funded by the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska‐Curie Grant (ReSurface; grant no. 797148) as well as the National Institutes of Arthritis, and Musculoskeletal and Skin Diseases (NIAMS), and the Harvard Stem Cell Institute.
Written By: Dr Rosanne Raftery, Lecturer in Clinical Anatomy

New Research: Mobilizing Endogenous Progenitor Cells
New research led by iEd Hub lecturer Rosanne Raftery, is now online in Advanced Healthcare Materials. The paper describes the use of [...]

iEd Hub Course Brochure 2024/2025
iEd Hub is pleased to publish our 2024/2025 course brochure. The brochure provides information on the broad range of upskilling and reskilling [...]

MSc in Medical Device Development
Applications are open for the MSc. Medical Device Development! The MSc in Medical Device Development has been co-developed between the Engineering faculty at [...]

Harnessing the Power of Stem Cells to Repair Damaged Joints
Harnessing the power of stem cells to repair damaged joints Every day, the human body is busy sealing cuts, mending micro-damage to our [...]

New Research: Mobilizing Endogenous Progenitor Cells
New research led by iEd Hub lecturer Rosanne Raftery, is now online in Advanced Healthcare Materials. The paper describes the use of [...]

iEd Hub Course Brochure 2024/2025
iEd Hub is pleased to publish our 2024/2025 course brochure. The brochure provides information on the broad range of upskilling and reskilling [...]

MSc in Medical Device Development
Applications are open for the MSc. Medical Device Development! The MSc in Medical Device Development has been co-developed between the Engineering faculty at [...]

Harnessing the Power of Stem Cells to Repair Damaged Joints
Harnessing the power of stem cells to repair damaged joints Every day, the human body is busy sealing cuts, mending micro-damage to our [...]


